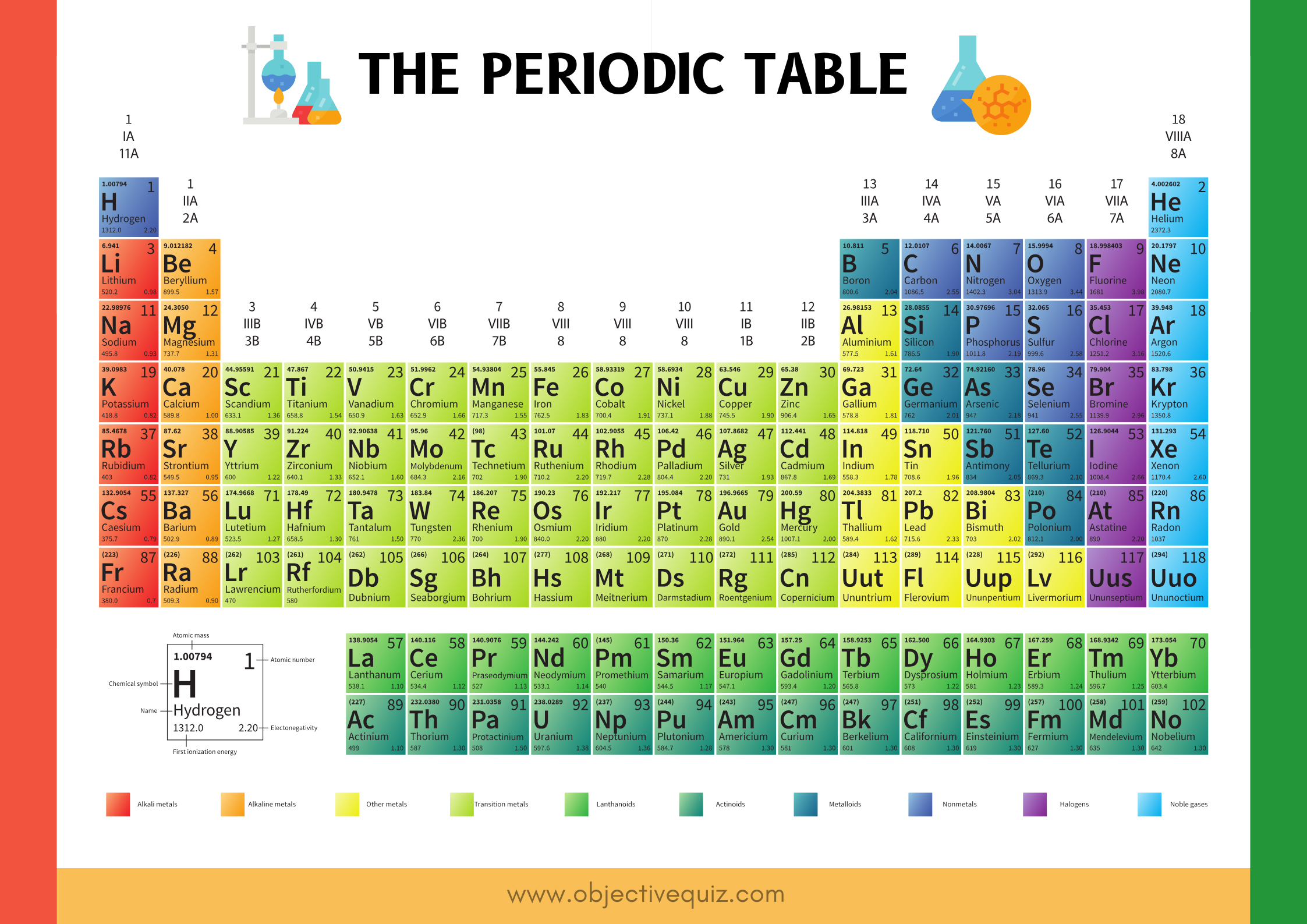
The Periodic Table of Elements in Chemistry
The periodic table is the most important chemistry reference. The periodic table displays the symbols and the names of the elements along with information about the structure of their atoms.The periodic table consist of 118 elements. The periodic table is made up of rows of elements and columns.
- A row is called a period.
- A column is called a group.
The Chemist used properties of elements to sort them into groups.
- An element is identified by its chemical symbol.
- The number above the symbol is the mass number.
- The number below the symbol is the atomic number.
What does the periodic table include?
The standard form of the table includes periods (usually horizontal in the periodic table) and groups (usually vertical). Elements in groups have some similar properties to each other. There is no one single or best structure for the periodic table but by whatever consensus there is, the form used here is very useful. The periodic table is a masterpiece of organised chemical information.
Who invented the periodic table?
Dmitri Mendeleev invented the first widely recognized periodic table in 1869 by arranging elements by increasing atomic mass and properties, leaving gaps for undiscovered elements. The "modern periodic table," which uses atomic number instead of atomic mass, was developed later by Henry Moseley.
In the modern periodic table elements are arranged in order of increasing atomic number.
Periodic Law states: When elements are arranged in order of increasing atomic number, there is a periodic repetition of their physical and chemical properties.
How the Elements are arranged in the Periodic Table
The elements can be grouped into three broad classes based on their general properties. Three classes of elements are Metals, Nonmetals, and Metalloids. Across a period, the properties of elements become less metallic and more nonmetallic.
Groups
Columns of elements are called groups or families. Elements in each group have similar but not identical properties. For example, lithium (Li), sodium (Na), potassium (K), and other members of group IA are all soft, white, shiny metals. All elements in a group have the same number of valence electrons. Four chemical groups of the periodic table: alkali metals (IA) alkaline earth metals (IIA), Halogens (VII), Noble gases (VIIIA).
Period
Each horizontal row of elements is called a period. The elements in a period are not alike in properties. In fact, the properties change greatly across even given row. The first element in a period is always an extremely active solid. The last element in a period, is always an inactive gas.